Development and Promotion of Non-POPs Alternatives to DDT





Dichlorodiphenyltrichloroethane (DDT) is an organochlorine insecticide synthesized by the Austrian Chemist Othmar Zeidler, in 1874. However, its insecticidal property was discovered only in 1939 by Paul Muller, for which he was awarded Nobel Prize in 1948 for Physiology or Medicine. The chemical structure of DDT permits several isomers, amongst, the ortho, para-DDT (o,p’-DDT), and para, para-DDT (p,p’- DDT), are the important isomers. However, the insecticidal property is predominantly due to the p,p-DDT isomer.
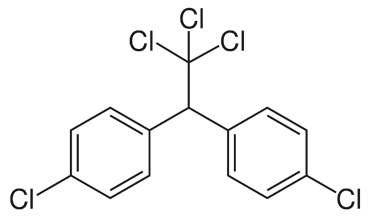
Chemical structure of p,p-DDT
Commercially, the term DDT is used for products containing predominantly of p,p'- DDT, but, other isomers of DDT such as o,p-DDT, and their degradation products such as p,p'- and o,p'-DDD (dichlorodiphenyldichloroethane) and p,p' and o,p'-DDE (dichlorodiphenyldichloroethylene) may also be present in appreciable quantities (about 20-25 %). For public health purpose, DDT is formulated as “technical grade” (should contain a minimum of 70 % p,p′-DDT) as per the specification of the World Health Organization (WHO, 2009). Typically, technical grade DDT also contains smaller amounts of other compounds such as o,p′-DDT (15-21 %), p,p′-DDD (also known as p,p′- TDE, 4 %) and o,p′-DDE (WHO, 2009). The other isomeric forms and the degradation products of DDT may add to the insecticidal action of “technical grade” DDT (Muller, 1948). For public health purposes, DDT is formulated in several different forms such as aerosols, dustable powders, emulsifiable concentrates, granules and wettable powders.
Physicochemical properties and Environmental behaviour of DDT
DDT is immobile in most soils and sediments. DDT does not undergo degradation easily and residues remain for decades i.e., persistent. Its half-life (i.e., the time required for 50% degradation under natural environmental conditions) varies between 15-20 years (Ricking and Schwarzbauer, 2012). Biotic and abiotic processes such as runoff, volatilization, photolysis and biodegradation contribute its breakdown in the environment very slowly. Its breakdown products in the soil environment are DDE and DDD, which are also highly persistent and have similar chemical and physical properties as that of the parent molecule.
DDT and its degradation products are semi-volatile in nature (slowly evaporate from soil to the atmosphere due to persistence in the atmosphere). In the atmospheric air, DDT can travel long distances (long-range transboundary movement) and undergo deposition where it is never produced or used (Schenker et al., 2008). DDT and its degradation products are sparingly soluble in water but highly soluble in fats and lipids i.e., lipophilic. Due to this property, DDT and its metabolites undergo enrichment in fatty tissues and accumulate in the food chain, i.e., bioaccumulation. Also, progressive magnification of DDT concentration at all tropic levels of food chain occurs, leading to serious ecological and adverse human health problems (IARC 2015).
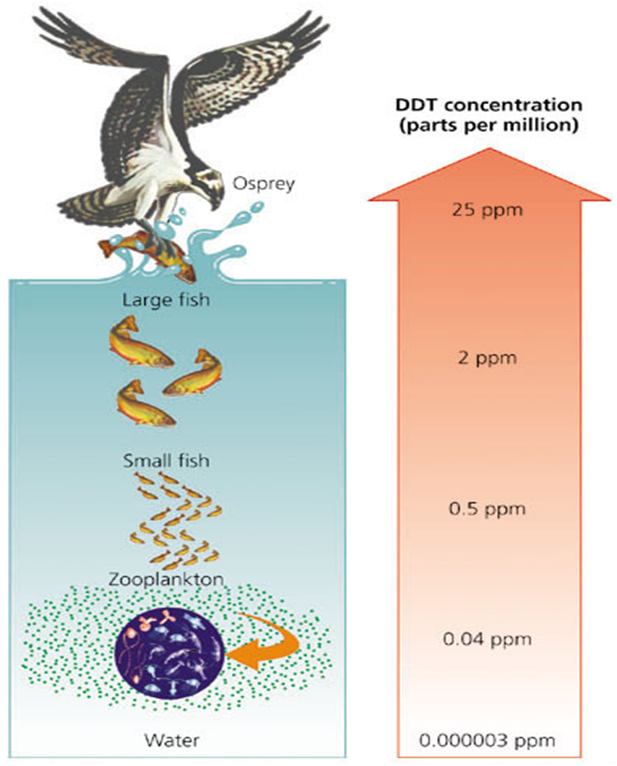
Magnification of DDT concentration along tropic levels of food chain